
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
Select Language
![]() November 05, 2022
November 05, 2022

Pictured: Enhanced "artificial dermis" sample. Photo courtesy of the Second Hospital of Zhejiang Medical University
"At present, the enhanced artificial dermis has successfully developed samples, and the large animal experiment has been successful, and will soon enter the clinical trial stage. It is expected that the fastest 3 years, it is expected to apply domestic artificial dermis in clinical." Second Affiliated Hospital of Zhejiang University Han Chunmao, director of the Burns Department, felt that after 18 years, the “artificial leather” jointly developed by his team and a number of research institutes has finally reached the stage of clinical trials, which means that China is expected to break the monopoly of “artificial leather” production technology in the international market. Reduce the production cost of domestic PGA (polyethyl acetate) and its copolymer fibers and related products.
It is reported that because PGA and its copolymer fiber products are mainly monopolized by several foreign companies, China currently uses "artificial leather" in the United States, Japan and other countries in the clinical practice of burns. The price is very expensive, and a piece of palm-sized "artificial leather" is sold. The price is equivalent to the price of an iphone7, and the demand for wound transplantation in a large area of burn patients is very large (tens of thousands of pieces), and the high price is unbearable for ordinary patients.
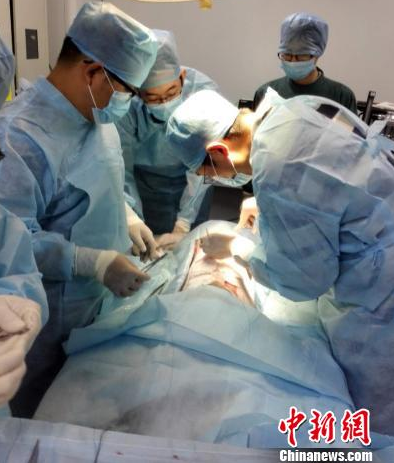
Pictured: Han Chunmao team is conducting a live animal experiment of “artificial dermis”. Photo courtesy of the Second Hospital of Zhejiang Medical University
How to break the monopoly, reduce the cost of products, and let more patients enjoy the benefits brought by scientific research and innovation? This is a question that Han Chunmao has been thinking about in clinical practice. 18 years ago, Han Chunmao began to cooperate with Zhejiang University and devoted himself to the research of tissue engineering skin. One of the important contents is the research and development of artificial leather.
In 2002, Han Chunmao's "Research on Vascularization of Collagen Porous Membrane" project laid a good foundation for the study of vascularization of "artificial dermis". In 2013, the team applied to the Zhejiang Provincial Natural Science Foundation for the “Optimized Construction of Enhanced Dermal Regeneration Template and Mechanism Research for Promoting Burn Wound Healing” project, which was successfully passed.

Pictured: Bio 3D printer. Photo courtesy of the Second Hospital of Zhejiang Medical University
In 2016, Han Chunmao teamed up with Zhejiang University, China Textile Science Research Institute and other institutions and enterprises to undertake the national 13th Five-Year Key R&D project - "Glycolide with in situ tissue induction and repair regeneration function" The research and development of its composite fiber reinforced leather substitutes, with a total funding of more than RMB 60 million, aims to break the international monopoly on artificial skin production technology and significantly reduce the production cost of domestic “artificial leather”.
"The most classic product of artificial dermis is Integra in the United States. It takes three weeks to survive effectively after transplantation. The large animal living experiment suggests that the 'artificial dermis' developed by our team can only be vascularized in about ten days. Cut in half, and the faster you survive, the faster you can rebuild the barrier and close the wound.” Han Chunmao said that this is crucial to improving the success rate of treatment.
It is reported that the project is progressing smoothly. The “artificial dermis” product has passed the formal test and is entering the clinical trial phase. Han Chunmao team also purchased a new bio-based 3D printer, aiming at the development of more advanced bio-repair materials. Once the experiment is successful, the “artificial dermis” can be used immediately, and the individual treatment plan can be truly provided for patients. (China News Network)
The above is the China's intensive artificial dermal products entering clinical trials are expected to break international monopoly we have listed for you. You can submit the following form to obtain more industry information we provide for you.
You can visit our website or contact us, and we will provide the latest consultation and solutions
Send Inquiry
Most Popular
lastest New
Send Inquiry

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.